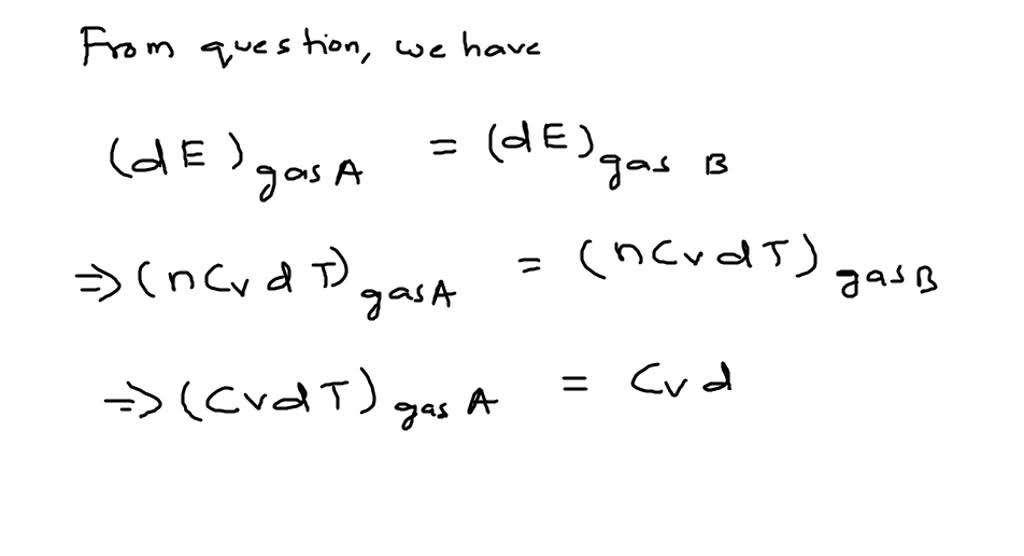
SOLVED:Two monatomic ideal gases A and B are at the same temperature. If 1.0 g of gas A has the same internal energy as 0.10 g of gas B, what are (a)
At 27^(@)C, two moles of an ideal mono-atomic gas occupy a volume V. The gas expands adiabatically to a volume 2V. Calculate (a) final temperature of the gas (b) change in its

One Kg of a diatomic gas is at a pressure of 8 × 10^4 N/ m^2 . The density of the gas is 4 kg/ m^3 . The energy of the gas
30.10: The Potential-Energy Surface Can Be Calculated Using Quantum Mechanics - Chemistry LibreTexts

OneClass: 6. Chapter: 2 Lesson: 1 Question: Green light of wavelength 516 nm is absorbed by an atomic...

SOLVED: Two monoatomic ideal gases A and B are at the same temperature: If 1.25 g of gas A has the same internal energy as 0.125 g of gas B. (a) Calculate